Compliance questionnaire
Please note that EMBRACE II is no longer open for new centers, as the compliance questionnaire closed on May 1st 2018.
The first step towards EMBRACE II accreditation is a web-based evaluation of current practice at each centre through a compliance questionnaire.
The compliance criteria are:
- Treatment of >10 patients per year qualifying for enrolment in EMBRACE II
- Both EBRT and BT are performed in the centre
- Routine use of IMRT or VMAT
- Routine use of daily IGRT with bony fusion
- Routine use of MRI guided IGABT with applicator in situ (at least for first fraction)
- Routine use of the combined intracavitary-interstitial technique when needed (~20-50% of patients)
Based on the evaluation of the compliance questionnaire, the study coordinators will decide if a centre is ready to proceed with the dummy run. The dummy run will ensure that the contouring and treatment planning is consistent with protocol requirements.
Dummy run
The dummy run includes a registration phase as well as submission of contouring and dose planning for evaluation. Based on this, the study coordinators will evaluate if the centre is ready to participate in EMBRACE II.
Contouring cases for BT and EBRT are accessed through the Cambridge Cancer Medicine Online platform (ccmo.co.uk). Contouring is performed online using the Addenbrooke’s Contouring Tool (ACT). All principal investigators are required to undertake contouring according to the EMBRACE II guidelines as outlined in chapters 9 and 10. Non-PIs are also encouraged to submit contours. Instructions, case descriptions, diagnostic information and contouring guidelines are all available online in CCMO.
An EBRT planning dataset can be downloaded from the CCMO platform for evaluation of dose planning. Centres should perform IMRT or VMAT dose planning using their own TPS according to the EMBRACE II guidelines in chapter 9. The DVH results should be uploaded into CCMO for evaluation using the reporting sheet provided.
When contouring and dose planning has been performed, the centre can proceed with the registration of 5 consecutive patients (within 6 months) in a registration database. The registration database is a copy of the EMBRACE II database with online reporting of
1) Status at diagnosis, 2) Status at brachytherapy and 3) Treatment and DVH parameters.
Centres are also required to submit screen dumps of EBRT and BT contours, cartoons documenting clinical examination at diagnosis and at BT, and dose plans.
Training & Education
EMBRACE II is unique in providing a programme of continuous education for all study participants. The programme is hosted on CCMO and includes
- Training cases for EBRT and BT contouring
- Dose planning exercises for EBRT and BT
- Step-by-step guides
- Quizzes
All educational content is for self-learning. The study coordinators may monitor participant responses and use the information to create additional resources as required.
Overview of participating centers
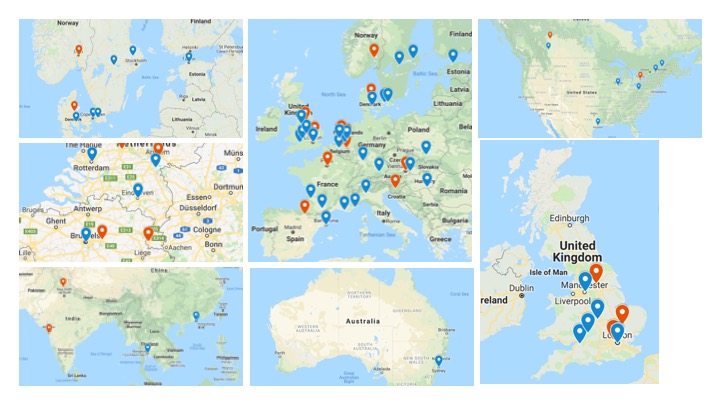
Red pins indicate centers finished with the accreditation and accruing patients.
Blue pins indicate centers still in the accreditation process (as of March 2019).